Background:Multiple myeloma (MM) mostly affects older adults. Although representing around 10% of all cases, patients ≤ 50 years old suffer from the highest number of years lost due to their disease. MM in young patients occurs during their most productive years of life, resulting in a significantly higher personal, familial, professional, and economic burden. Young MM patients are currently underrepresented in clinical trials due to small numbers overshadowed in cohorts of older patients. Their disease characteristics and outcomes following modern treatments remain poorly understood (Tanguay M et al., Current Oncol 2023). The purpose of this study is to report disease characteristics, treatments, and outcomes of young MM patients treated in Canada.
Methods:This is a retrospective study using the Canadian Myeloma Research Group Database, which is a repository of real-world patient data reflecting 17 of the major MM academic centers across Canada. All patients 18-50 years old with newly diagnosed MM between January 1, 2010 and July 1, 2022 were included. High-risk (HR) cytogenetics were defined as either del17p, t(4;14) or t(14;16). Survival analyses were performed using the Kaplan-Meier method.
Results:Among the 6,604 patients newly diagnosed with MM during the study period, 504 (7.6%) were aged between 18-50 years, and included in this analysis. Within this young group, 87 (17.3%) were 18-40 years old and 417 (82.7%) were between 41-50 years. MM was diagnosed in 301 (59.7%) patients between 2010 to 2015, and in 203 (40.3%) patients between 2016 to 2022. The median age at diagnosis was 45.9 years (range 25.6 to 50). Male patients represented 57.7% of the cohort. IgG was the most common isotype in 251 (49.8%) cases, while 115 (22.8%) patients had light chain disease. In 375 patients with available data, 16% were R-ISS 1, 73.1% R-ISS 2, and 10.9% R-ISS 3. Lytic lesions, elevated creatinine, anemia, hypercalcemia, and high LDH were respectively present in 46.8%, 15.5%, 44%, 18.8%, and 18.7% of patients. HR cytogenetics were present in 23.7% of patients, with del17p in 11.5%, t(4;14) in 14.9%, and t(14;16) in 5.1%; while 1q+ and del1p were detected in 27.9% and 15.3%, respectively. Twelve (2.4%) patients were diagnosed with plasma cell leukemia.
First-line treatment was proteasome inhibitor (PI)-based in 462 (91.7 %) and immunomodulatory (IMiD) agent-based in 7 (1.4 %); 18 (3.6%) received both a PI and an IMiD and 17 (3.4%) were treated with other modalities. Among all patients, 453 (89.9%) underwent autologous stem cell transplant (ASCT) during their disease course. Some patients did not receive ASCT for the following reasons: early death before ASCT in 10 (2%) cases, lost to follow-up prior to ASCT in 13 (2.6%) cases, deemed ineligible for ASCT or patient declined in 24 (4.8%) cases, and participation in a clinical trial not involving ASCT in 1 (0.2%) case. Out of the entire cohort, allogeneic-SCT (allo-SCT) was performed in only 11 patients (2.2%), including 3 (0.6%) who received allo-SCT alone without ASCT. Among patients who received maintenance post ASCT, 93.6% were treated with a lenalidomide-based regimen. Median duration of the different maintenance treatments was 21.2 months.
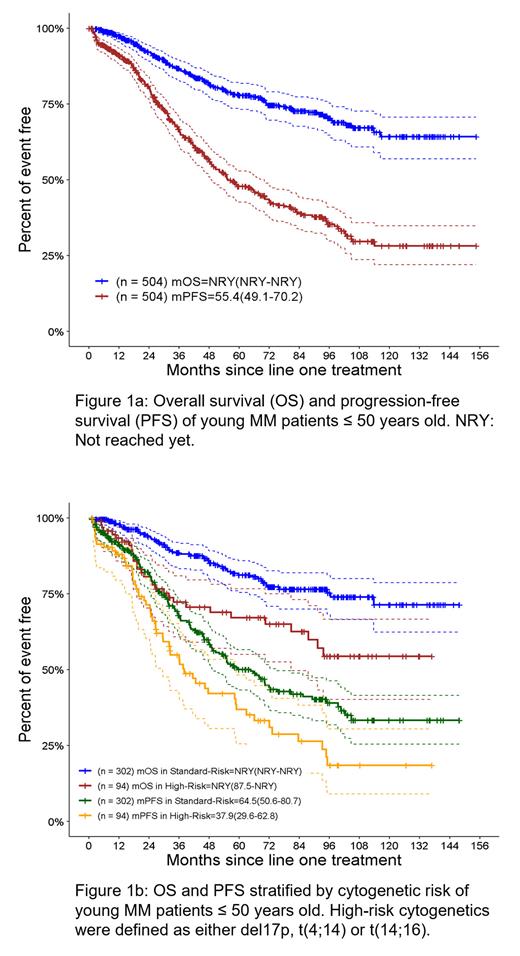
With a median follow-up of 51.9 months (range 0.9 to 154), median PFS (progression-free survival) was 55.4 months (95% CI 49.1-70.2), while median OS (overall survival) was not reached. The 5-year OS and PFS were 78% (95% CI 74-82) and 48% (95% CI 43-53), respectively, whereas the 10-year OS and PFS were 64% (95% CI 58-71) and 28% (95% CI 22-35), respectively (Figure 1a). Median PFS for patients with any HR cytogenetics was 37.9 months (95% CI 29.6-62.8) vs 64.5 months (95% CI 50.6-80.7) for patients with standard-risk cytogenetics (p=0.004) (Figure 1b). We observed no difference in disease characteristics, treatments, and outcomes after comparing cohorts aged 18-40 years old vs 41-50 years old.
Conclusions: This is among the largest real-world cohorts of young MM patients ≤ 50 years old treated with modern therapies ever reported. Most patients were R-ISS 2 (73.1%), 23.7% had known HR cytogenetics, and the majority (91.7%) received a PI-based first-line treatment. Outcomes of specific subgroups are under analysis and will be presented. Although the OS was not yet reached in this young population, the median PFS of only 55.4 months highlights the urgent need to develop more effective treatments to induce deeper and more durable responses.
Disclosures
Roy:Sanofi: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; ExCellThera: Patents & Royalties, Research Funding. Reece:Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Millennium: Research Funding; Amgen: Consultancy; Sanofi: Honoraria; Pfizer: Honoraria; GSK: Honoraria. Venner:Janssen: Honoraria; BMS: Honoraria; Pfizer: Honoraria; AbbVie: Honoraria; Sanofi: Honoraria; Forus: Honoraria; GSK: Honoraria. White:Amgen: Honoraria; Antengene: Honoraria; BMS: Honoraria; Forus: Honoraria; GSK: Honoraria; Janssen: Honoraria; Karyopharm: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Chu:AstraZeneca: Honoraria; BMS/Celgene: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Miltenyi: Research Funding. Jimenez-Zepeda:BMS: Honoraria; Merck: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Song:GSK: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; BMS: Honoraria; Forus: Honoraria; Amgen: Honoraria; Gilead: Honoraria. McCurdy:Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GSK: Honoraria; Forus therapeutics: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Mian:Amgen: Honoraria; GSK Awards: HHS Research Early Career Award from Hamilton Health Sciences Foundation: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Forus: Honoraria; Roche: Current equity holder in publicly-traded company; Janssen: Honoraria, Research Funding; Celgene / BMS: Honoraria. Sebag:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Bergstrom:BMS: Honoraria, Research Funding; Janssen: Honoraria. Stakiw:Janssen: Honoraria; Forus: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria. Reiman:AstraZeneca: Consultancy, Honoraria, Research Funding; Regeneron: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Kotb:Takeda: Honoraria; Karyopharm: Current equity holder in private company; Forus: Honoraria; Celgene: Honoraria; BMS: Honoraria; Sanofi: Honoraria, Research Funding; Amgen: Honoraria; Pfizer: Honoraria; Janssen: Honoraria; Merck: Honoraria, Research Funding; Akcea: Honoraria. Aslam:AbbVie: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Kaedbey:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Louzada:Janssen: Honoraria; BMS: Honoraria; GSK: Research Funding; Forus: Honoraria. LeBlanc:Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; FORUS Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; BMS: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal